In STEM class the second round of the year, we had learned about Chemical bonding. In chemical bonding, there are Covalent bonding, Ionic bonding, and Metallic bonding. For this writing, I would only describe about Covalent bonding and Ionic Bonding.
Ionic Bond: All compounds form when atoms of different elements share or transfer electrons. In water, the atoms share electrons. In some other compounds, called ionic compounds, atoms transfer electrons. The electrons actually move from one atom to another. When atoms transfer electrons in this way, they become charged particles called ions. The ions are held together by ionic bonds. Ionic bond form only between metals and nonmetal. When it is Ionic bond an element will gain or lose the electron.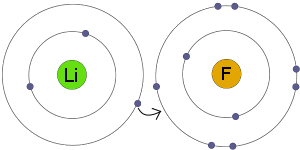
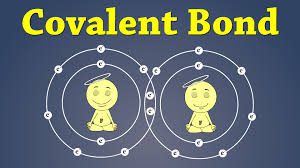
Covalent bond is the force of attraction that holds together two atoms that share a pair of electrons. The shared electrons are attracted to the nuclei of both atoms. Covalent bonds form only between atoms of nonmetals. The two atoms may be the same or different elements. If the bonds form between atoms of different elements, a covalent compound forms. Covalent bonds form because they give atoms a more stable arrangement of electrons.